Rules for the naming of Binary Ionic compound. Ionic compounds composed of two elements.
An ionic compound that contains only two elements one.
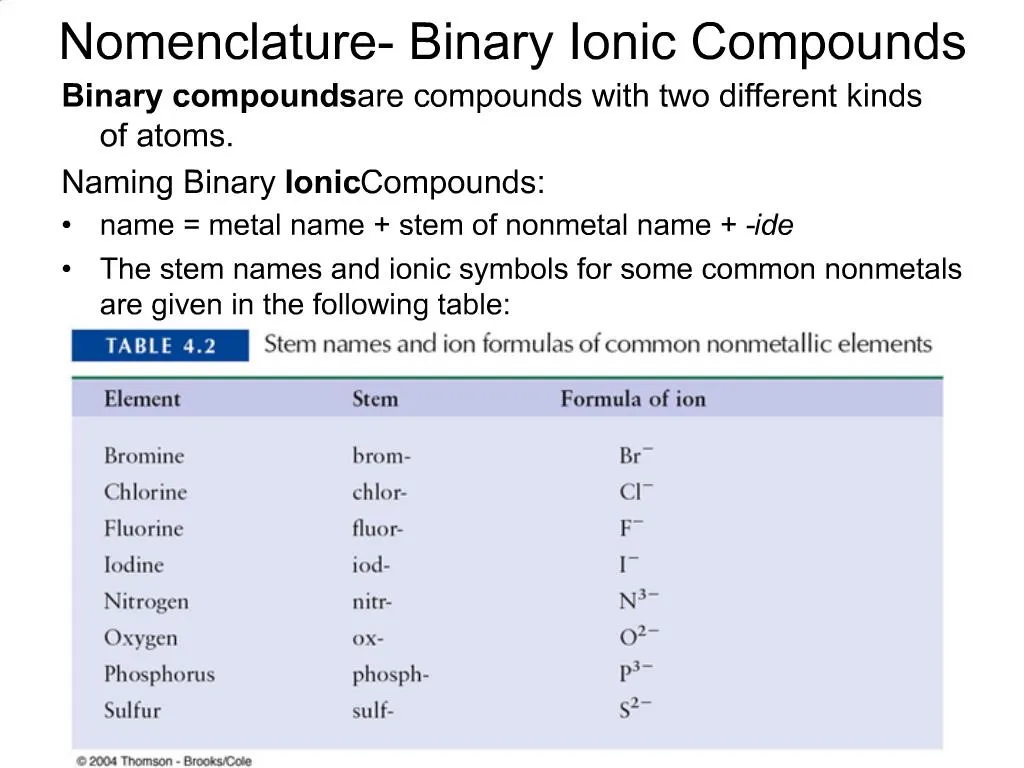
. The naming of ionic compounds is dependent upon the type of ionic molecule formed from alkali metals alkaline earth metals or transition metals. The anion is named after using the suffix -ide. 2O Binary since there are only two types of atoms.
CHCl 3 Not binary or diatomic. CuOH2 SnC2H3022 Sn3PO42 PbNO32 NaHCO3 FeCO3 Fe2SO43 CuClO32 Sc2Cr2O73 Hg2CN2 Part IV. If the negative ion is a NON-METAL element àchange its ending suffix to ide.
Students often have difficulty remembering all the rules for naming ionic and covalent compounds. Up to 24 cash back How to Write Chemical Names for Binary Ionic Compounds Chemical name has 2 parts to represent both ions in the compound Step 1. By using an inquiry approach students analyze patterns and create their own rules helping them to not only remember the rules better but also to have a deeper understanding of the way compounds are named.
Name of Cation metal Base Name of anion nonmetal -ide Format For naming binary compounds with transitional metals. NAMING BINARY TERNARY AND QUATERNARY COMPOUNDS. Compound name cation name anion name Examples.
If the metal atom is Transition element then the roman numeral is included after the metal name to indicate the oxidation number of the metal. Keys to Naming Binary Ionic Compounds. The order followed to name in binary compounds is such that the cation is named first and then the anion.
First write the full name of cation. Nomenclature of Binary Molecular Compounds Containing Nonmetals Observing the Patterns. Name the negative ion do not capitalize Step 3.
Al2O3 aluminum oxide MgCl2 magnesium chloride. CobaltIII oxide ironIII chloride copperII bromide lithium arsenide chromiumIII sulfide leadIV iodide potassium nitride ironII phosphide Use the rules you have determined to write the names of the following binary ionic compounds. CH 4 Binary since there are only two types of atoms.
For example KCl an ionic compound that contains K and Cl- ions is named potassium chloride. Names of binary compounds end in _____. Collectively these elements comprise the Main Group elements.
Chemical nomenclature naming compounds also follows this pattern. Name the positive ion write it in full usually a metal Step 2. Name the Cation First The cation or the positively charged particle always begins the compounds name.
Name the metal the cation as it appears on the Periodic Table. Name of Cation metal Charge in parentheses Base Name of anion nonmetalide. MnF 2 Ni 3P 2 PbO 2 Cs 2S ScCl 3 MgI 2.
When a metal and non-metal form an ionic molecule the metal will retain the element name and the non-metal will taken the suffix -ide. This set includes a bunch of binary compounds and it includes the compounds with transitional metals. While naming the anion consider the elements root name and add the suffix -ide Binary Ionic Compound Binary ionic compounds are salts which consist of only 2 elements.
Use the rules you have determined above to write the formulas of the binary molecular compounds listed below. Fe2O3 ironIII oxide Fe2O3 ferric oxide. Metals combine with nonmetals to give ionic compounds.
Can you write formulas for binary ionic compounds. The binary ionic compounds name begins with the full name of the metal followed by the name of. Format for naming Binary Compounds.
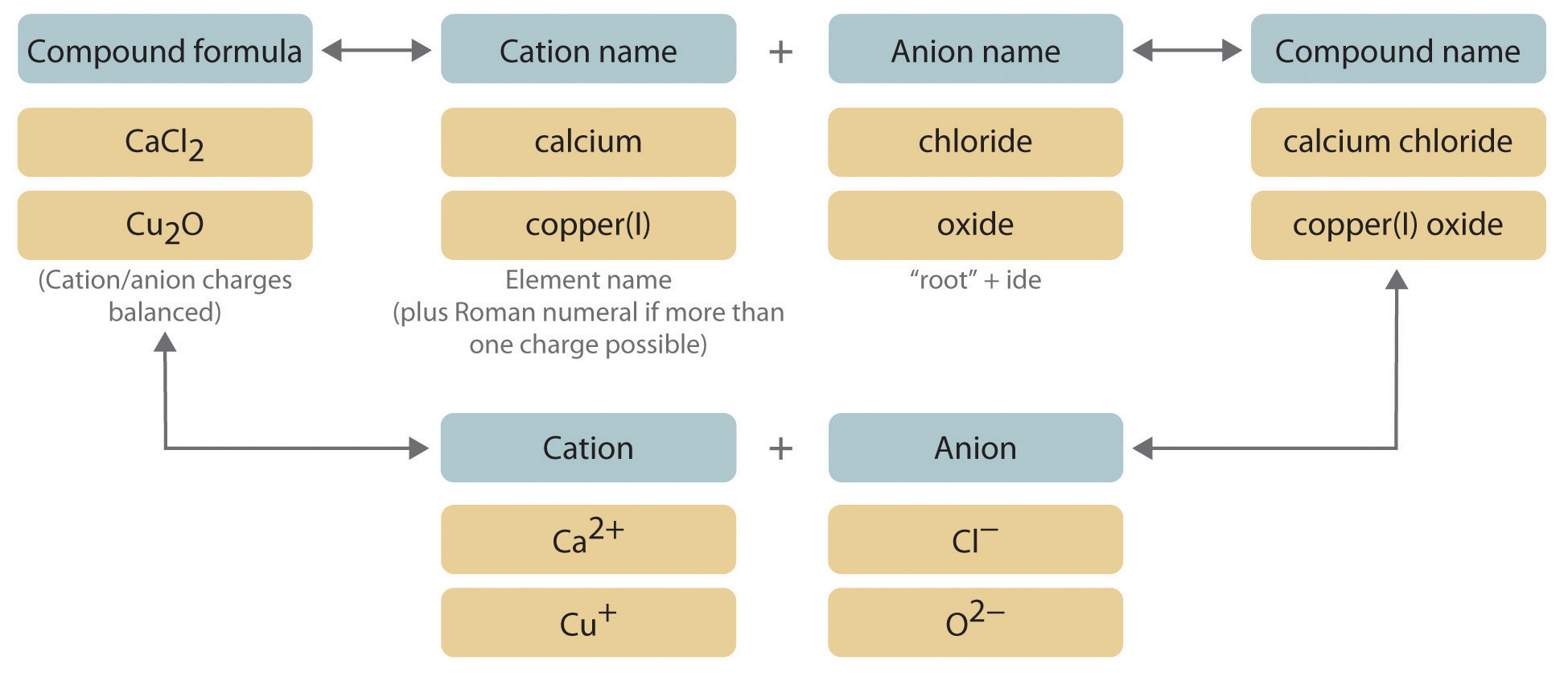
Cupric chloride CuCl cuprous chloride. From the periodic table consider cation with a fixed oxidation state. For binary ionic compounds ionic compounds that contain only two types of elements the compounds are named by writing the name of the cation first followed by the name of the anion.
Can you name binary ionic compounds. When naming binary ionic compounds name the cation first specifying the charge if necessary then the nonmetal anion element stem -ide. If the anion is bromine the name will be bromide and so on.
2 When the anion name ends in -ite the acid name is the stem of the anion with suffix -ous followed by the word acid. Na Sodium Mg 2 Magnesium Al 3 Aluminum For the non-metal the anion write the name on the Periodic Table and then replace the ending with ide. Up to 24 cash back 1 In the formula of binary ionic compounds the metal cation always appears first followed by the non- metal anion positive before negative.
Either way the binary ionic naming process will apply. Nomenclature of Binary Ionic Compounds Composed of Main Group Elements This activity focuses on the nomenclature rules for ionic compounds composed of two different elements from groups IA IIA IIIA IVA VA VIA and VIIA on the periodic table of the elements. Ions that consist of o single atom.
The stem of the anion has the suffix -ic and is followed by the word acid. 5 rows Formulas of Binary Ionic Compounds. CaCl 2 Calcium chlor ine Calcium chlor ide AlN Aluminum nitrogen Aluminum nitri de.
Binary Metal to Non-Metal. Binary Ionic Compounds formula contains one metal and one nonmetal. Compounds of metals with halides or oxygen are usually ionic solids which have a lattice connecting the cations and anions held together with electrostatic forces.
1 When the name of the anion ends in -ide the acid name begins with the prefix hydro-. Binary ionic compounds are named by writing the name of the _____ followed by the name of the _____. Do NOT use prefixes to indicate how many of.
The cation name is placed first when naming ionic compounds.

Chemistry 101 Naming Binary Ionic Compounds Youtube

Forming And Naming Ionic Compounds Type 1 And 2 Binary Compounds Ppt Download

Solved Part Ii Binary Ionic Compounds Containing Chegg Com

Naming Ionic Compounds A Guided Inquiry Exercise

Naming Binary Ionic Compounds Rules Examples Expii
5 7 Naming Ionic Compounds Chemistry Libretexts

Ppt Nomenclature Binary Ionic Compounds Powerpoint Presentation Free Download Id 721132

0 comments
Post a Comment